SCIENCE
Aberrant immune system activity is an effector of nervous system disease. Blocking such activity will ameliorate the cause of symptoms and progression in many devastating neurodegenerative and neuropsychiatric disorders.Our Science
MindImmune scientists bring together deep knowledge of both the nervous and immune systems to discover previously unrecognized immune effector mechanisms underlying brain disease. The team is operationalizing this knowledge to create its facile drug discovery engine. Our flagship program may herald a breakthrough therapeutic for Alzheimer’s disease.
'nipping Alzheimer's disease in the blood'
MindImmune has discovered that an innate immune cell from the blood enters the brain and causes the synaptic damage at the core of Alzheimer’s symptoms and progression. MindImmune is developing a novel antibody therapeutic that ameliorates this synaptic damage simply by preventing these immune cells from entering the brain.
Alzheimer’s disease is a chronic inflammatory condition, akin to atherosclerosis, fibrosis, and psoriasis. In each case, the immune system becomes inappropriately activated and then the inflammatory response fails to resolve.
In Alzheimer’s disease this inappropriate immune response manifests as innate immune first responder cells infiltrating into the brain, where these cells cause damage to synapses. This synaptic damage underlies both the cognitive symptoms and the relentless progression of Alzheimer’s disease.
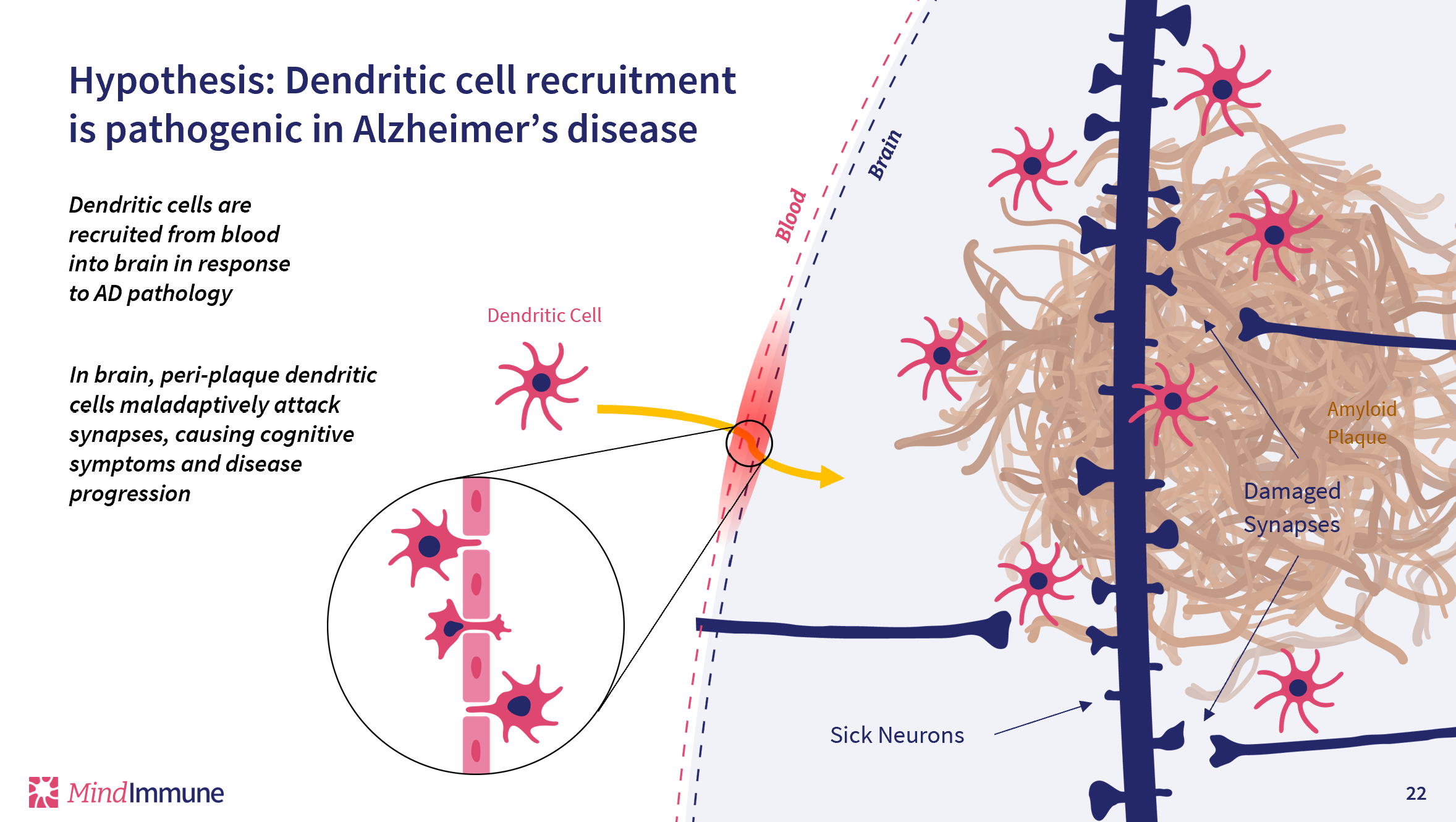
MindImmune has identified the key innate immune cells that infiltrate the Alzheimer’s brain to damage synapses. MindImmune is developing an antibody therapeutic to block this infiltration, thereby ameliorating the synaptic attack.
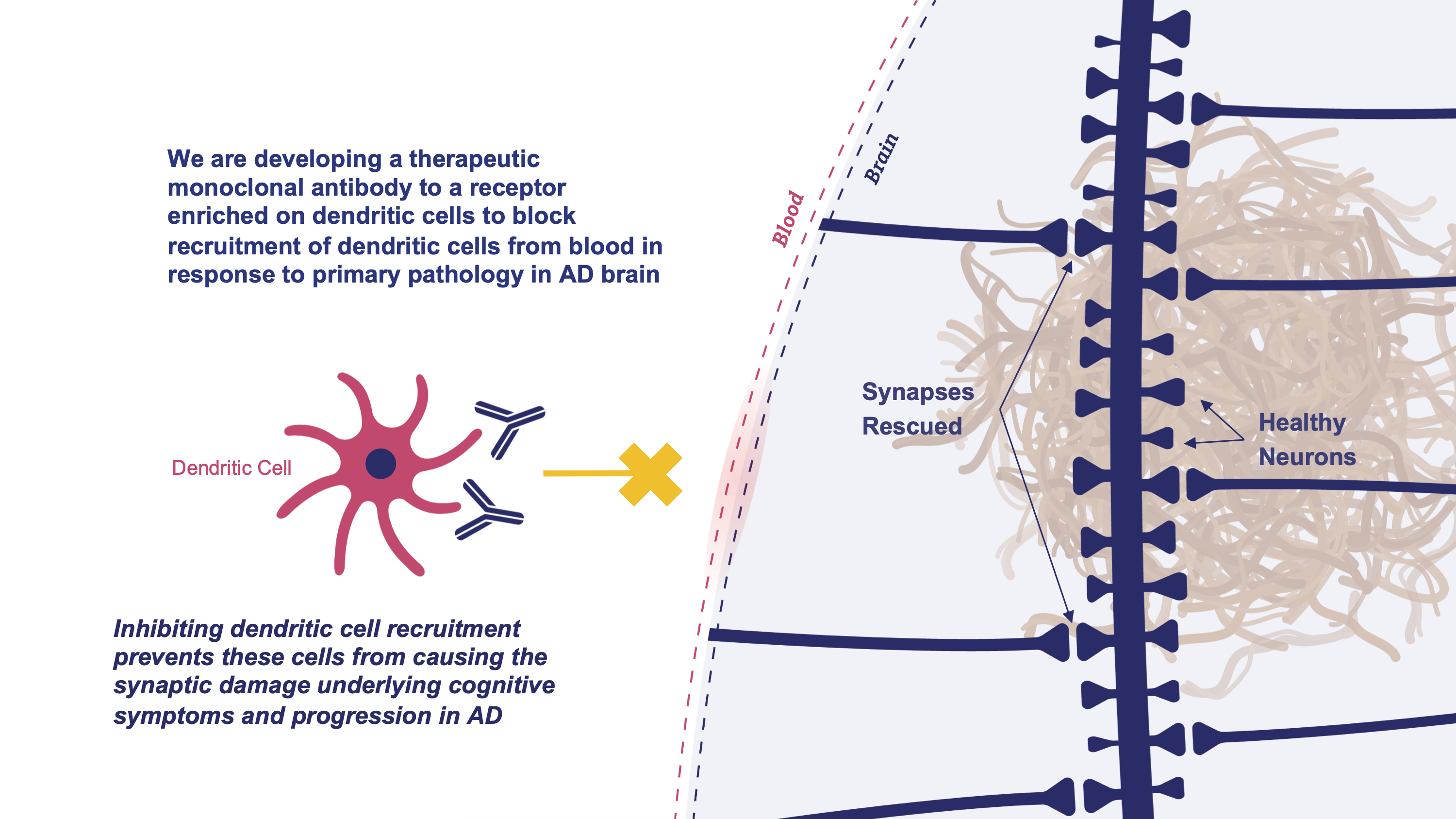
Blocking these immune cells from entering the brain prevents the damage they do to synapses. Synaptic damage is the core pathology in Alzheimer’s disease, causing the day-to-day cognitive symptoms as well as the insidious progression of the disease. Thus, blocking immune cell recruitment may improve cognitive symptoms and slow the progression of this devastating disease.
MindImmune’s therapeutic strategy for Alzheimer’s disease is applicable to a range of neurodegenerative disorders as well as to the other chronic inflammatory conditions.
We are developing a pipeline of therapeutics tailored to the aberrant immune system activity that drives these diseases.
Collaborations
MindImmune is affiliated with the George & Anne Ryan Institute for Neuroscience with laboratories at The University of Rhode Island. The Ryan Institute is at the forefront of public/private collaborative efforts to develop new therapies to treat neurodegenerative diseases.
Our Collaborators:
Jessica Alber, PhD
Research Assistant Professor, George & Anne Ryan Institute for Neuroscience. University of Rhode Island
Robert Berman, MD
Biotech Neuroscientist and Entrepreneur
Stefan Brocke, MD, PhD
Associate Professor of Immunology, University of Connecticut
Peter J. Snyder, PhD
Vice President for Research & Economic Development, and Professor of Biomedical Sciences, University of Rhode Island
Excessive reservations and paralyzing despondency have not helped the sciences to advance nor are they helping them to advance, but a healthy optimism that cheerfully searches for new ways to understand, as it is convinced that it will be possible to find them.
— Alois Alzheimer
